

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR-TRC-12002577
暂停或中断
/
/
/
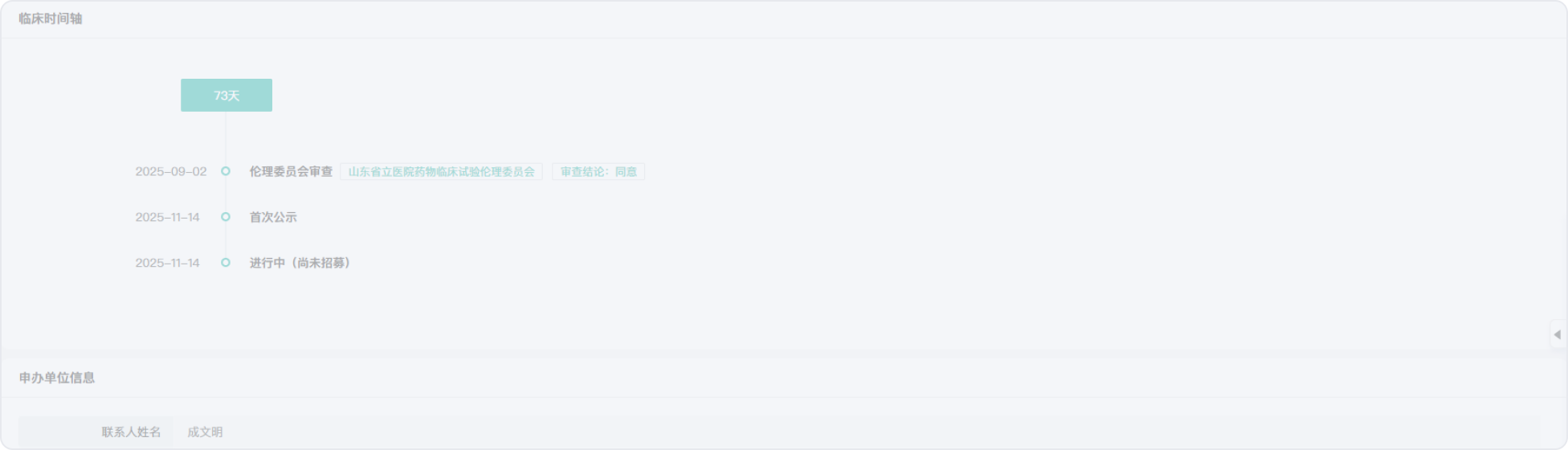
2012-10-19
/
/
Heart Failure
心臟肌肉收縮力調節對己接受同步治療但沒有預期改善的心臟衰竭病人的治療效果的研究
心臟肌肉收縮力調節對己接受同步治療但沒有預期改善的心臟衰竭病人的治療效果的研究
心臟肌肉收縮力調節對己接受同步治療但沒有預期改善的心臟衰竭病人的治療效果的研究
非随机对照试验
其它
Randomized 1:1
/
Impulse Dynamics
/
80;0
/
2005-07-06
2013-07-05
/
1. Heart failure (HF) patients remain in New York Heart Association Class III or IV who have received cardiac resynchronization in form of biventricular pacemaker or biventricular cardiovertor-defibrillator devices. 2. Have been classified as non-responder of CRT as defined by absence of reduction of left ventricular end-systolic volume reduction of less than 15% after 3months of therapy. 3. Have been treated with optimal anti-HF medications with doses not varying by more than 50% for a minimum of 4 weeks, e.g. diuretics, Angiotensin Converting Enzyme Inhibitors or Angiotensin Receptor Blocker, ?-blockers, etc.;
请登录查看1. Subjects with severe symptomatic heart failure who would qualify for heart transplant, or who have recently (within 2 weeks) been hospitalized and treated with intravenous inotropic agents or intravenous diuretics or whose baseline VO2, max is known to be <10 ml O2/min/kg; 2. Subjects who have a potentially correctible cause of heart failure, such as valvular heart disease or congenital heart disease; 3. Subjects who have clinically significant angina pectoris, consisting of angina during daily life (i.e., Canadian Cardiovascular Society Angina score of II or more), an episode of unstable angina within 30 days of enrollment, or angina and/or ECG changes during exercise testing performed during baseline evaluation; 4. Subjects without an ICD who have a documented history of sustained VT, or who have an indication for an ICD and are not scheduled for ICD implantation at the time of OPTIMIZER III implantation; 5. Subjects who have an ICD who have had appropriate ICD firing during the past one month; 6. Subjects who have a clinically significant amount of ambient ectopy, defined as more than 8,900 PVCs per 24 hours on baseline Holter monitoring; 7. Subjects who have chronic atrial fibrillation or chronic atrial flutter; 8. Subjects whose exercise tolerance is limited by a condition other than heart failure (e.g., angina, COPD, peripheral vascular disease, orthopedic or rheumatologic conditions); 9. Subjects who are unable to participate in a 6-minute walk; 10. Subjects who are scheduled for a CABG or a PTCA procedure, or who have undergone a CABG procedure within three months or a PTCA procedure within one month of enrollment; 11. Subjects who have had a myocardial infarction within three months of enrollment; 12. Subjects who have mechanical tricuspid or aortic valves; 13. Subjects who have a prior heart transplant; 14. Subjects who are participating in another experimental protocol; 15. Subjects who are unable to provide informed consent.;
请登录查看Impulse Dynamics
/
西湖生物医药2026-02-21
Htology2026-02-21
MedTrend医趋势2026-02-21
肝脏时间2026-02-21
新通药物2026-02-21
数字医疗2026-02-21
医药笔记2026-02-21
医药笔记2026-02-21
医药笔记2026-02-21
药明康德2026-02-21