

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR1800014444
尚未开始
/
/
/
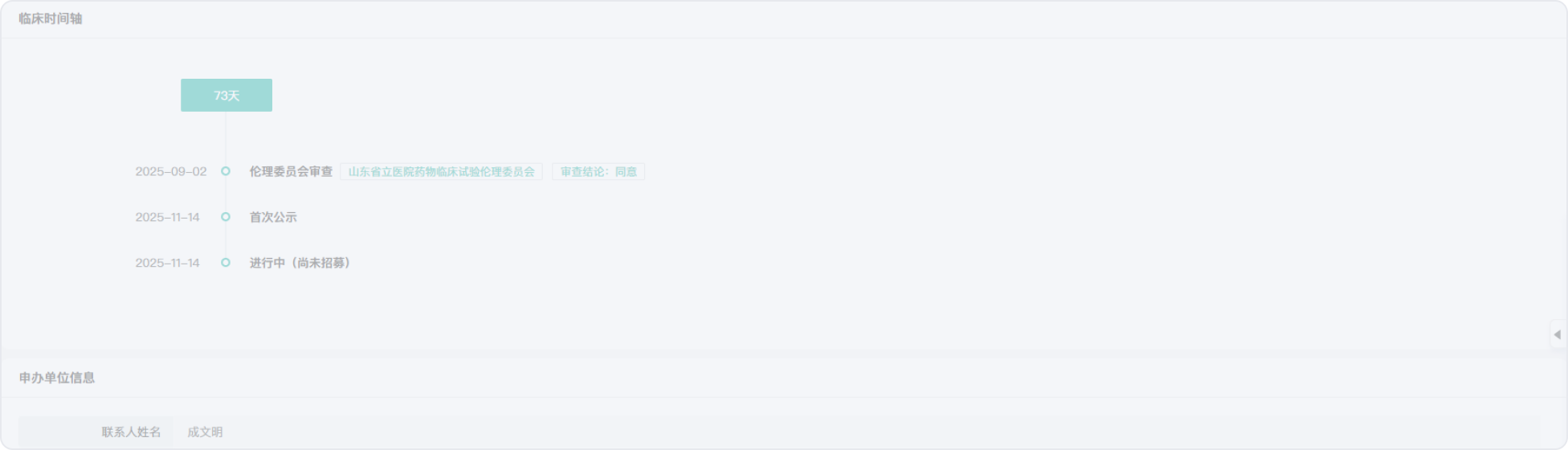
2018-01-15
/
/
Myofascial pain
Effect of prolotherapy with trigger point dextrose/levobupivacaine mixture injection on myofascial pain syndrome: A randomized controlled trial
Effect of prolotherapy with trigger point dextrose/levobupivacaine mixture injection on myofascial pain syndrome: A randomized controlled trial
To investigate the effect of trigger point injection with dextrose/levobupivacaine on myofascial pain syndrome.
随机平行对照
Ⅲ期
computer generated randomization
/
Self sponsor
/
25
/
2018-02-01
2019-12-31
/
1. Patient aged 18 years old or above 2. Patient with musculoskeletal pain in trapezius muscle which has met the Travell and Simons criteria 3. Informed consent will be obtained from the patient in compliance with ICH-GCP.;
请登录查看1. Pregnancy 2. Patient on anticoagulant therapy 3. Patient with local skin infection 4. Allergy to study medication (25% dextrose + 0.25% levobupivacaine) 5. Mentally incapable patient 6. More than 3 Trigger points within the trapezius 7. Any form of injection/ acupuncture / dry needle therapy to MTrPs within 3 months;
请登录查看Ng Hiu Ying
/
求实药社2026-03-05
生物制品圈2026-03-05
医药观澜2026-03-05
医药观澜2026-03-05
河北省干细胞中心2026-03-05
生物制药小编2026-03-05
一度医药2026-03-05
炎明生物Pyrotech2026-03-05
药明康德2026-03-05
疑夕随笔2026-03-04