

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR2100045116
结束
/
/
/
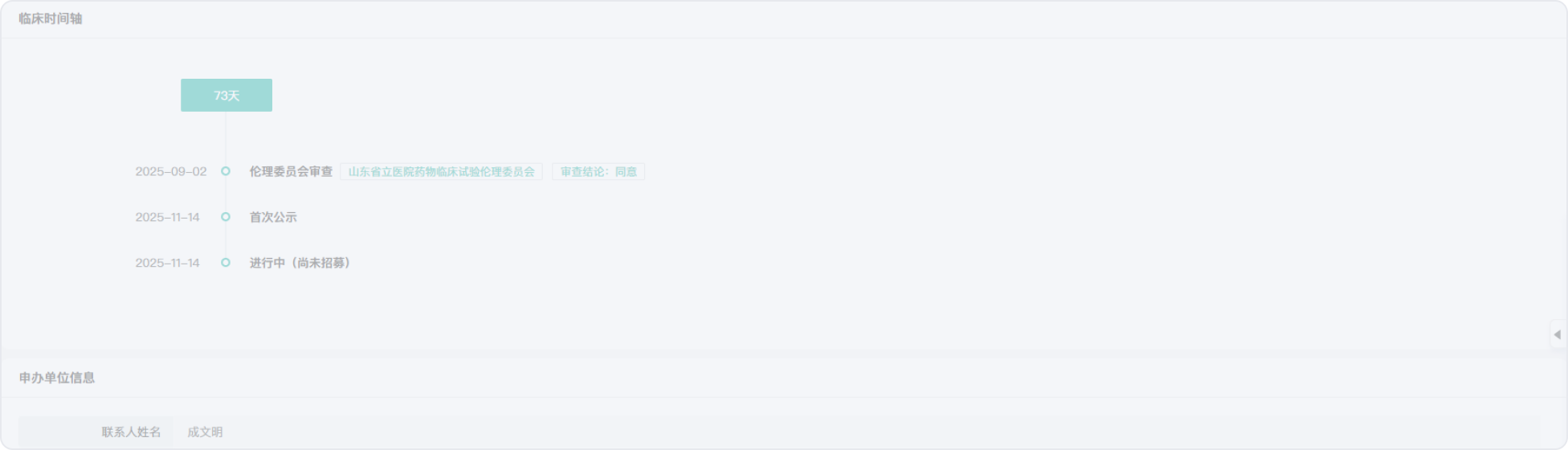
2021-04-07
/
/
Chronic kidney disease
Efficacy of Vitamin D Supplementation and Physical Activity in Improving Musculoskeletal Health in Individuals with Chronic Kidney Disease
Efficacy of Vitamin D Supplementation and Physical Activity in Improving Musculoskeletal Health in Individuals with Chronic Kidney Disease
Aims of the study: To assess the improvement in musculoskeletal health in individuals with chronic kidney disease having 25 OH vitamin D deficiency by giving vitamin D3 (cholecalciferol) and increased physical therapy. Primary Objectives: To compare improvement in objective measure of musculoskeletal health as assessed by hand grip measure in individuals with chronic kidney disease randomized to either vitamin D3 alone or vitamin D3 along with targeted physical therapy. To compare the improvement in physical functioning in individuals with chronic kidney disease randomized to either vitamin D3 alone or vitamin D3 along with targeted physical therapy based on subjective questionnaires. To compare differences in musculoskeletal health as measured by change in muscular strength and physical functioning in individuals with chronic kidney disease randomized to either vitamin D3 alone or vitamin D3 along with targeted physical therapy. Secondary Objectives: To assess the improvement in serum 25OH vitamin D levels among patients with Chronic Kidney disease with vitamin D3 supplementations.
随机平行对照
Ⅰ期
Randomization will be performed via computer generated random numbers by a statistician. A block randomization strategy with individual blocks with varying multiples of 4 will be used to periodically balance the assignment in the 2 study arms. Randomization lists will be stratified by Hospitals (AKUH and kidney
Not stated
None
/
28;18
/
2012-01-01
2016-12-31
/
1. Patients aged 20 to 60 years; 2. CKD stage 2-4; 3. Patients with CKD and having creatinine of >=1.7 mg/dL for men and >=1.4 mg/dL for women will be screened; 4. Vitamin D deficient subjects (<=20 nmol/L) (32); 5. Able to provide written informed consent.;
请登录查看1. Patients with any transplant (due to use of immune-suppression); 2. Patients with CKD 5 or ESRD; 3. Patients receiving dialysis; 4. Patients who are under treatment for Vitamin D deficiency; 5. Patients who have some sort of known vitamin D deficiency related bone disease by history; 6. Subjects already maintained on steroids; 7. Patients Who Have Been On (25 Or 1, 25, OH Vitamin D Supplementation for Vitamin D Repletion- I/M in last One Year; 8. Patients with known chronic inflammatory disease, malignancy; 9. Subjects who cannot perform physical activity due to any disability or limiting condition; 10. Patients with known depression; 11. Patients with calcium and phosphorus product of > 70 mg/dl.;
请登录查看None
/
求实药社2026-03-05
生物制品圈2026-03-05
医药观澜2026-03-05
医药观澜2026-03-05
河北省干细胞中心2026-03-05
生物制药小编2026-03-05
一度医药2026-03-05
炎明生物Pyrotech2026-03-05
药明康德2026-03-05
疑夕随笔2026-03-04