

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR1800016098
结束
/
/
/
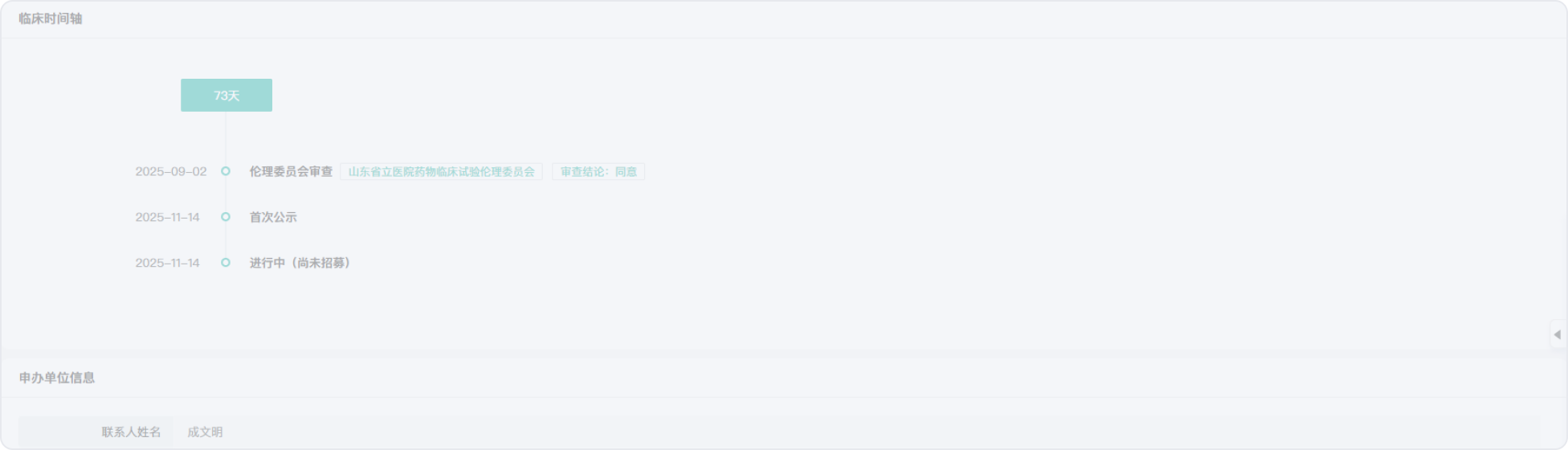
2018-05-02
/
/
Patients undergoing elective cardiac surgery (CABG, with/without valvular interventions) Pre-frail and Frail patients
擇期心臟手術前復康計劃研究: 隨機對照試驗
擇期心臟手術前復康計劃研究: 隨機對照試驗
In patients undergoing primary elective cardiac surgery, does prehabilitation during the two months waiting period before surgery compared to no prehabilitation improve postoperative quality of recovery?
随机平行对照
Ⅲ期
Randomized
Single-blind for Investigator/research team
Department of Anaesthesia and Intensive Care, The Chinese University of Hong Kong; Local research grant by the General Research Fund (Pending)
/
164
/
2018-07-03
2023-10-31
/
1. Adults undergoing elective primary isolated coronary artery bypass grafting, aortic valve repair/replacement, mitral valve repair/replacement, or combined coronary artery bypass/valve procedures; 2. Prefrail to moderately frail patients with a Clinical Frailty Score of 4-6 at the time of accepting surgery at the outpatient cardiothoracic surgical clinic; 3. Patients with an estimated 8 or more weeks of surgical waiting list time; 4. Agreement on the suitability of the patient for prehabilitation with the attending cardiothoracic surgeon.;
请登录查看1. Patients with unstable or recently unstable cardiac syndrome (New York Heart Association Class IV, critical left main coronary disease, hospitalization for arrhythmias, congestive heart failure or acute coronary syndrome before randomisation); 2. Patients with severe left ventricular obstructive disease (severe aortic or mitral stenosis, dynamic left ventricular outflow obstruction); 3. Redo cardiac surgery; 4. Contraindications for prehabilitation (those with cognitive deficits unable to comply with study procedures, physical limitations precluding rehabilitation and inability to regularly attend outpatient prehabilitation sessions).;
请登录查看香港中文大學麻醉與重症監護部
/
求实药社2026-03-05
生物制品圈2026-03-05
医药观澜2026-03-05
医药观澜2026-03-05
河北省干细胞中心2026-03-05
生物制药小编2026-03-05
一度医药2026-03-05
炎明生物Pyrotech2026-03-05
药明康德2026-03-05
疑夕随笔2026-03-04