

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR2500108626
结束
/
/
/
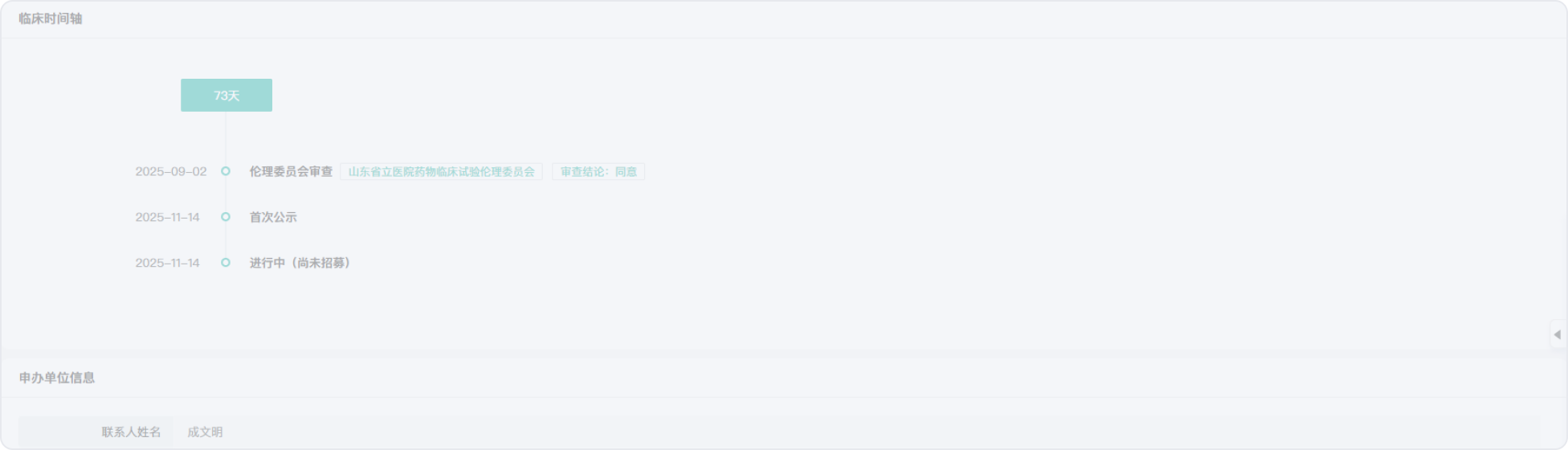
2025-09-02
/
/
Children with disabilities
A Multilevel Implementation Study for Physical Activity Promotion in Children with Disabilities
A Multilevel Implementation Study for Physical Activity Promotion in Children with Disabilities
(1) To promote both physical (e.g., physical activity levels, physical fitness) and psychosocial (e.g., enjoyment, quality of life) health among children with disabilities). (2) To advance professional competence practice of PE teachers and practitioners working with children with disabilities. (3) To enhance knowledge and participation of family on adapted physical activity. (4) To develop an effective and sustainable adapted physical activity model that empowers children with disabilities to become active and healthy in their school and family settings.
单臂
其它
None
None
The Hong Kong Jockey Club
/
1200
/
2020-01-21
2024-08-31
/
A total of 40 local special schools which enroll children with four disability types including vision impairment, hearing impairment, physical disability, and intellectual disability (mild or moderate levels) would be include. The target population was Hong Kong children with vision impairment, hearing impairment, physical disability, and intellectual disability who are at 6-18 years.;
请登录查看Exclusion criteria include medical conditions that limit participation in physical activity.;
请登录查看The Chinese University of Hong Kong
/
GSK中国2026-01-30
上海市浦东新区生物产业行业协会2026-01-30
罕见病信息网2026-01-30
中肽生化2026-01-30
Farsightpharma睿诺医疗科技2026-01-30
丁香园 Insight 数据库2026-01-30
一度医药2026-01-30
晨泰医药2026-01-30
医麦创新药2026-01-30
医药观澜2026-01-30