

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR-IIC-16010249
尚未开始
/
/
/
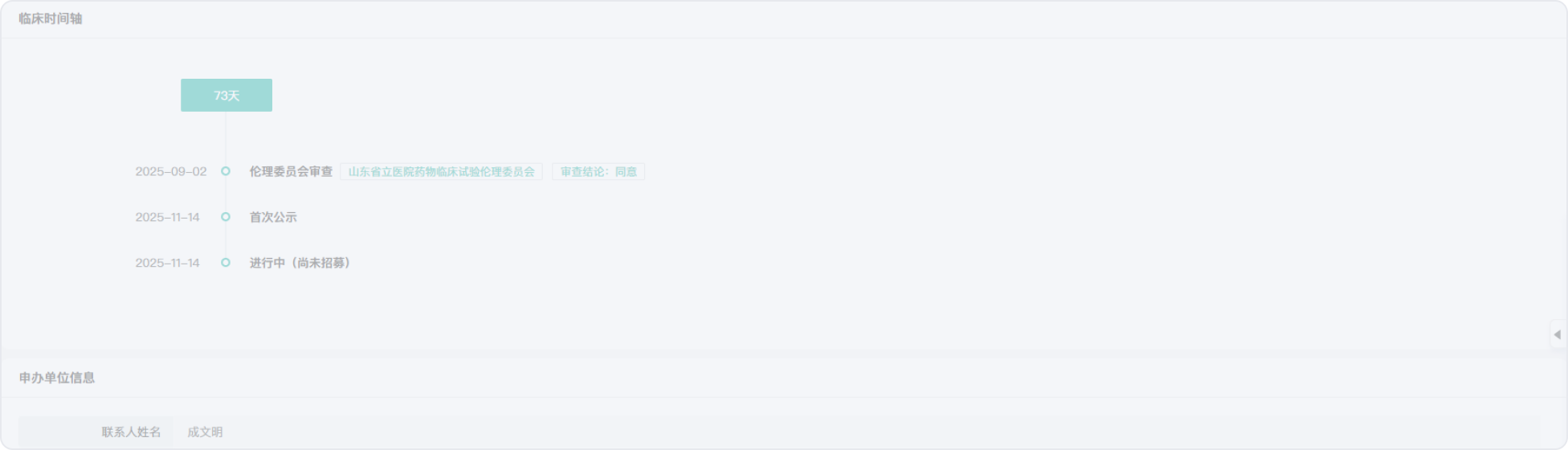
2016-12-22
/
/
卵巢多囊症
高能量聚焦式超音波(HIFU)治療無排卵性不孕的卵巢多囊症婦女之劑量研究
高能量聚焦式超音波(HIFU)治療無排卵性不孕的卵巢多囊症婦女之劑量研究
高能量聚焦式超音波(HIFU)治療無排卵性不孕的卵巢多囊症婦女之劑量
单臂
治疗新技术
NA
/
NA
/
30
/
1990-01-01
1990-01-01
/
1. Premenopausal women aged 18 to 40 years diagnosed with PCOS with anovulatory infertility; 2. Ability to give informed consent; 3. Body mass index <30 kg/m2; 4. Not on hormonal therapy (GnRH or combined oral contraceptive pills - COC) at the time of HIFU treatment; 5. No previous history of laparoscopic ovarian drilling or ovarian surgery; 6. Women with patent bilateral tubes; 7. Failure to achieve ovulation or pregnancy with incremental doses of clomiphene citrate; 8. Ability to give communicate clearly with a nurse or physician during HIFU treatment; 9. Women willing and able to comply with study requirements.;
请登录查看1. Pre-existing thyroid problem or hyperprolactinaemia which may cause anovulation; 2. Those with pre-existing compromised ovarian failure as evident by day 2 FSH >10IU/L or an AMH value of <0.75ng/ml before the treatment; 3. Pregnant or lactating; 4. Presence of active pelvic inflammatory disease, genital or extra-genital malignancy; 5. Extensive scarring along anterior lower abdominal wall (greater than 50% of area) or scar tissue or surgical clips in the direct path of the HIFU; 6. Unable to give consent or those who are unable for regular follow- ups after the study; 7. Those with an intrauterine device in situ; 8. Those with tattoos or metalic implants over their abdomen; 9. Male partner with moderate to severe male factor.;
请登录查看香港中文大學威爾斯親王醫院婦產科學系
/
求实药社2026-03-05
生物制品圈2026-03-05
医药观澜2026-03-05
医药观澜2026-03-05
河北省干细胞中心2026-03-05
生物制药小编2026-03-05
一度医药2026-03-05
炎明生物Pyrotech2026-03-05
药明康德2026-03-05
疑夕随笔2026-03-04