

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR-TRC-14004903
尚未开始
/
/
/
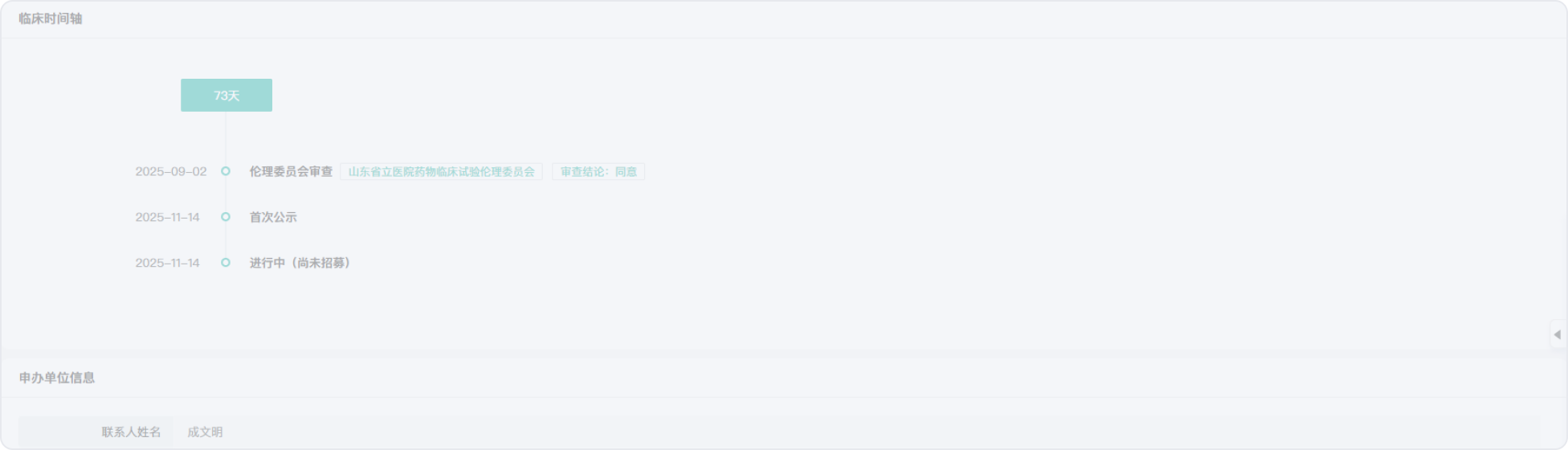
2014-06-27
/
/
HYPERTENSION
用雲端技術(IMPACT研究)去改善高血壓管理的隨機對照研究
用雲端技術(IMPACT研究)去改善高血壓管理的隨機對照研究
The primary aim of the study is to assess the effect of intervention relative to usual care on increasing the proportion of patients with controlled BP (defined as less than 140 mm Hg systolic and less than 90 mm Hg diastolic; less than 130 mm Hg systolic and less than 80 mm Hg diastolic in patients with diabetes or kidney disease) among hypertensive patients with suboptimal BP control at the 12 month follow-up clinic visit. 2.After 12 months, nurses will no longer monitor patients. However, patients will be followed up at 18 months to investigate the durability of the intervention effects, in terms of the proportion of patients with controlled clinic-based BP measurements; 3.The impact of the intervention on patient satisfaction and empowerment will also be assessed.
随机平行对照
Ⅳ期
Block stratified randomization for antihypertensive medication use (i.e., taking medication or not taking
Block stratified randomization for antihypertensive medication use (i.e., taking medication or not taking medication) will be used to ensure a balanced frequency of antihypertensive medication in each group. An independent statistician will generate a pre-determined random number table using Microsoft Excel. Research staff will use the randomization number generated to assign patients to one of the two arms of the trial. Nurse or HA clerk who measure the clinic-based BP will be blinded to the study design and intervention assignment. The allocation will be concealed from the health care assistants and professionals who carry out the annual assessment.
Health and Medical Research Fund (HMRF)
/
300
/
2014-07-14
2016-05-14
/
Eligible patients are: 1) age being 21 years or older; 2) have had at least two primary care encounters in the 12 months prior to screening with BP higher than 140/90 mm Hg in two separate occasions in the last 3 months; and 4) the average of two consecutive BP measurements taken at the research clinic screening visit being above the goal recommended by the 7th Joint National Commission for the Detection, Evaluation and Treatment of High Blood Pressure (JNC7).;
请登录查看Medical exclusion criteria include the following: the occurrence of known stage 4 or stage 5 kidney disease, acute coronary syndrome, coronary revascularization procedure or stroke within the past three months; known secondary causes of hypertension like coarctation of the aorta, pheochromocytoma, adrenocortical hypertension or renovascular hypertension; class III (marked limitation of physical activity) or IV (symptoms at rest) New York Heart Association classification; or known left ventricular ejection fraction less than 30%. Other additional exclusion criteria: unwillingness to be followed for a period of 18 months; pregnancy or intention to become pregnant in the next two years for females; participation in another clinical trial; spouse having already participated in this study; difficulty with communication in Cantonese; dementia, mental illness or any condition that would limit ability to give informed consent; or upper arm circumference longer than 18 inches (indicating BP cuff on telemonitoring device may not be accurate without modification).;
请登录查看PCCW
/
求实药社2026-03-05
生物制品圈2026-03-05
医药观澜2026-03-05
医药观澜2026-03-05
河北省干细胞中心2026-03-05
生物制药小编2026-03-05
一度医药2026-03-05
炎明生物Pyrotech2026-03-05
药明康德2026-03-05
疑夕随笔2026-03-04