

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR-TRC-09000743
结束
/
/
/
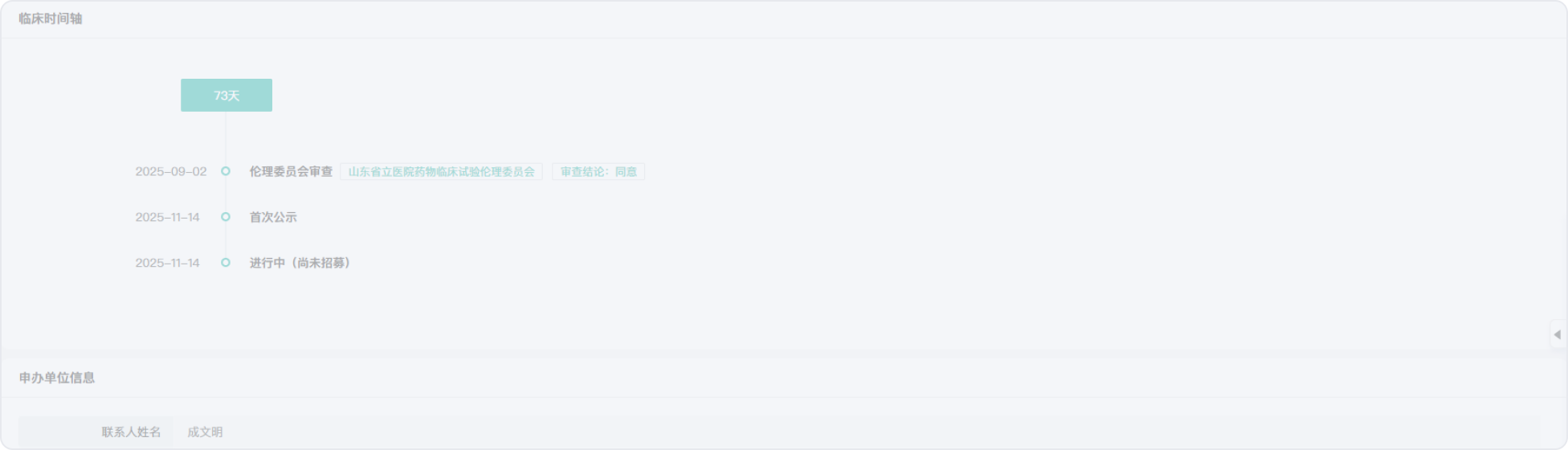
2010-05-04
/
/
Diabetic macular edema
The Safety and Efficacy of Combined Subtenon Triamcinolone and Grid Laser Photocoagulation as Primary Treatment for Diabetic Macular Edema - A Pilot Study
The Safety and Efficacy of Combined Subtenon Triamcinolone and Grid Laser Photocoagulation as Primary Treatment for Diabetic Macular Edema - A Pilot Study
The Safety and Efficacy of Combined Subtenon Triamcinolone and Grid Laser Photocoagulation as Primary Treatment for Diabetic Macular Edema
非随机对照试验
其它
NA
/
Department of Ophthalmology and Visual Sciences The Chinese University of Hong Kong
/
30
/
2007-06-10
1990-01-01
/
Inclusion criteria: 1. Aged 18 years or older; 2. Clinically significant macular edema (CSME) defined according to the ETDRS; 3. Macular edema of at least 250um involving the fovea, as documented on optical coherent tomogram; 4. Patients with best corrected visual acuity of better than 1.3 ETDRS logMAR units (equivalent to 20/400 on Snellen Chart); 5. Patients physically fit to receive treatment and comply with follow-up schedule; 6. Informed consent.;
请登录查看Exclusion criteria: 1. Ocular diseases other than cataract, diabetic retinopathy and refractive error; 2. Proliferative diabetic retinopathy; 3. Media opacities which affect fundus examination or OCT measurements; 4. Previous intraocular surgery except uncomplicated cataract extraction and posterior intraocular lens insertion more than 6 months prior to enrollment; 5. History of macular laser photocoagulation, intravitreal/ subtenon injection of triamcinolone acetonide or intravitreal injection of any vascular endothelial growth factor inhibitors; 6. History of fluorescein allergy; 7. Fellow eye visual acuity worse than 20/400.;
请登录查看The Chinese University of Hong Kong
/
求实药社2026-03-05
生物制品圈2026-03-05
医药观澜2026-03-05
医药观澜2026-03-05
河北省干细胞中心2026-03-05
生物制药小编2026-03-05
一度医药2026-03-05
炎明生物Pyrotech2026-03-05
药明康德2026-03-05
疑夕随笔2026-03-04