

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR-IPC-15007109
结束
/
/
/
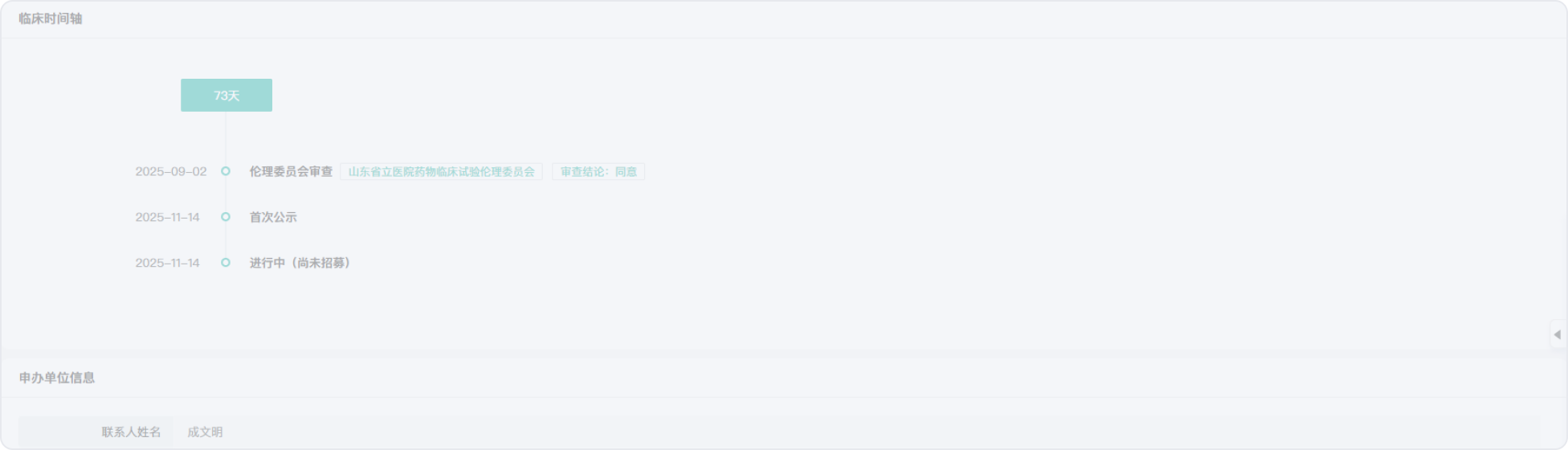
2015-09-18
/
/
功能性消化不良
電針灸聯合標準療法治療難治性功能性消化不良:實況隨機對照試驗及經濟學評價
電針灸聯合胃舒錠對比輪候電針灸聯合胃舒錠治療功能性消化不良:實況隨機對照試驗及經濟學評價
電針灸聯合胃舒錠與輪候電針灸聯合胃舒錠治療功能性消化不良患者的效果和成本效益比較
随机平行对照
治疗新技术
Blocked randomization will be used to allocate patients to the two groups, with random block sizes.
Not Applicable
Health andMedical Research Fund
/
132
/
1990-01-01
1990-01-01
/
1. Patients who have completed oesophagogastroduodenoscopy (OGD) with H. pylori negative results, or patients who has tested positive for H. pylori but have completed medication course the for the eradication of H. pylori; 2. Patients with symptoms that fulfill the reference standard for Functional Dyspepsia Postprandial Distress Syndrome, including the presence of either or both of the following symptoms once per week in the past 1 month:postprandial fullness, early satiety. In accordance to the pragmatic approach of this trial, this reference standard is chosen as it reflects presentations of FD patients in real-world clinical settings, increasing the external validity of future results; 3. Voluntary discontinuation of any conventional pharmacological treatments for their functional dyspepsia 2 weeks prior to enrolment, due to perceived ineffectiveness; 4. Hong Kong permanent resident; 5. 18 – 70 years of age.;
请登录查看Patients who fulfill any of the following criteria would be excluded. Criteria will be assessed through patient history, medical record review, or physical examination. 1. Documented diagnosis of esophageal or gastric disease, including esophagitis, gastroesophageal reflux disease (GERD), peptic ulcer, predominant heartburn or acid regurgitation in the past 1 month; 2. Current regular user of non-steroidal anti-inflammatory drugs, anti-depressants or anxiolytic drugs, as defined as daily use in the past 2 months; 3. Patients who had received major abdominal surgery; 4. Patients who are pregnant; 5. Patients who are wearing cardiac pacemaker; 6. Patients who are having underlying major physical illness such as malignancy and infections; 7. Patients are using any dose of PPIs or prokinetics two weeks prior to enrollment; 8. For patients with coexisting IBS as diagnosed by the Manning criteria, exclusion is applied if a patient considers abdominal or bowel symptoms, instead of dyspepsia, as their major complaint. Manning criteria for Irritable Bowel Syndrome (IBS) is positive in this trial when ≥ 4 of the following symptoms are present in the past month: Visible abdominal distension, Pain relieved by a bowel action, More frequent stools with the onset of pain, Looser stools with the onset of pain, Rectal passage of mucus, A sensation of incomplete evacuation.;
请登录查看香港中文大學賽馬會公共衛生與基層醫療學院
/
求实药社2026-03-05
生物制品圈2026-03-05
医药观澜2026-03-05
医药观澜2026-03-05
河北省干细胞中心2026-03-05
生物制药小编2026-03-05
一度医药2026-03-05
炎明生物Pyrotech2026-03-05
药明康德2026-03-05
疑夕随笔2026-03-04