

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR2200056203
正在进行
/
/
/
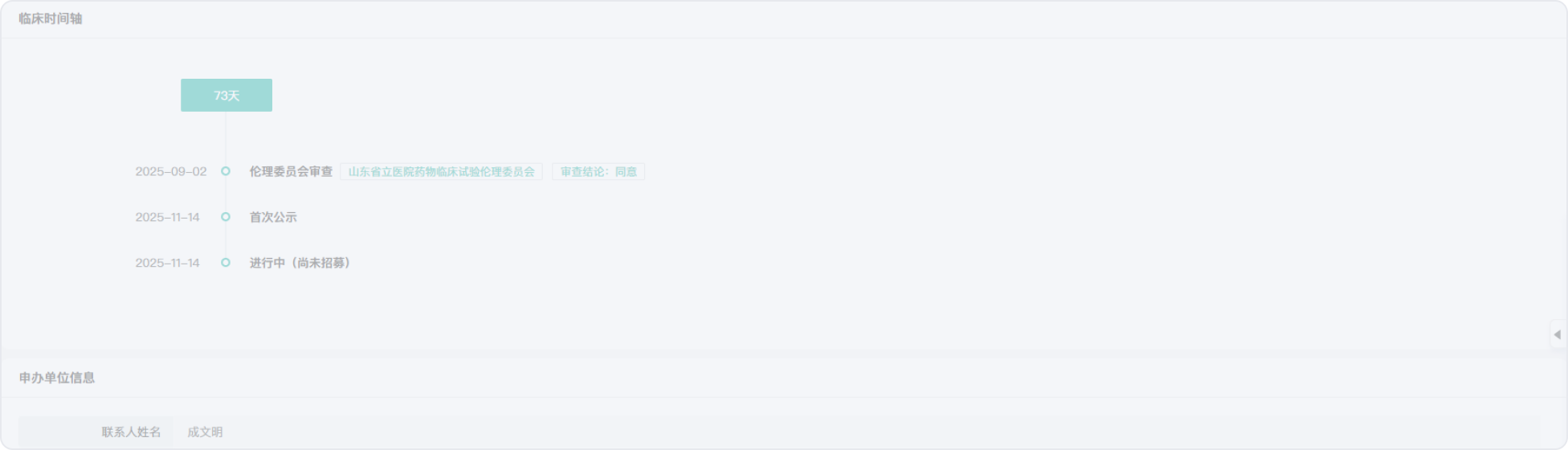
2022-02-01
/
/
多动症(ADHD)和自闭症(ASD)
Effect of Transcranial Direct Current Stimulation (tDCS) on Improving Inhibitory Control and Working Memory in Children with Attention-Deficit / Hyperactivity Disorder (ADHD) and comorbid Autism Spectrum Disorder (ASD) – A Randomised Controlled Trialutism Spectrum Disorder (ASD) – A Randomised Controlled Trial
Effect of Transcranial Direct Current Stimulation (tDCS) on Improving Inhibitory Control and Working Memory in Children with Attention-Deficit / Hyperactivity Disorder (ADHD) and comorbid Autism Spectrum Disorder (ASD) – A Randomized Controlled Trial
The primary objective of the study is to evaluate the effect of tDCS on inhibitory control and working memory in primary school-aged children with ADHD and comorbid ASD immediately after the intervention and 1-week post-intervention. The secondary objectives of the study include: 1. To compare the effect of tDCS on clinical symptoms of ADHD and ASD between the intervention group and control group at 1 week post-intervention. 2. To evaluate the safety profile of tDCS and describe any adverse events of tDCS in children with ADHD and comorbid ASD.
随机平行对照
其它
The subjects will be randomly allocated to the intervention group or the control group by a block randomization procedure with 1:1 group allocation. Allocation concealment will be adopted to prevent selection bias. The randomization sequence will be electronically generated.
Not stated
The Chinese University of Hong Kong
/
20
/
2021-11-29
2022-06-30
/
1. Age of 6 - 12 years old; 2. Right-handed; 3. With a diagnosis of ADHD and comorbid ASD made based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) by a Psychiatry specialist; 4. Able to understand spoken instructions in Cantonese and read written traditional Chinese; 5. Studying in mainstream school; 6. Currently not taking stimulant medications (Methylphenidate or Lisdexamfetamine), or agree to stop stimulant medications starting from 72 hours before the first stimulation and until one week after the last stimulation.;
请登录查看1. History of significant head trauma or skull defects; 2. Implanted with pacemakers, intracranial electrodes, defibrillators, or other metallic implants; 3. Neurological conditions including but not limited to seizure, stroke, intracranial tumors or aneurysms; 4. Sensory or motor impairments; 5. Documented history of Intellectual Disability; 6. Currently on psychotropics other than stimulants or other CNS drugs;
请登录查看nil
/
基石药业官微2026-02-16
一度医药2026-02-16
药明康德2026-02-16
医麦客2026-02-16
良医汇肿瘤资讯2026-02-15
国际眼科时讯2026-02-15
医麦客2026-02-15
药时空2026-02-15
石药集团2026-02-14
江苏康宁杰瑞生物制药有限公司2026-02-14