

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR2000031714
尚未开始
/
/
/
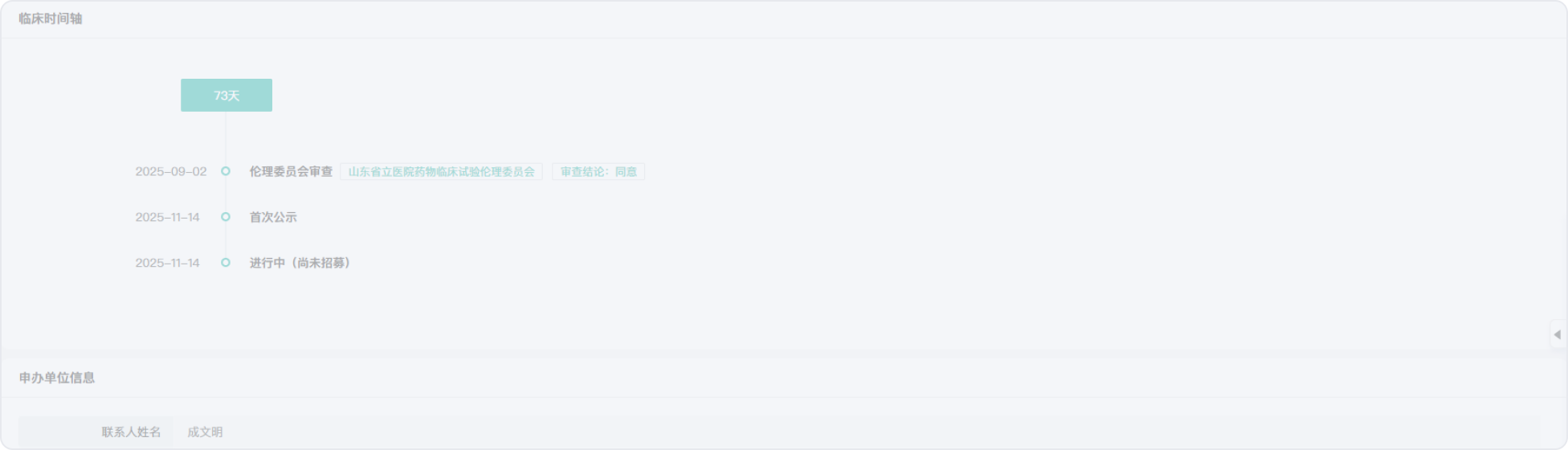
2020-04-08
/
/
Fracture neck of femur
Use of PENG block as a pre-operative analgesic tool in patients with fracture neck of femur - a feasibility study
Use of PENG block as a pre-operative analgesic tool in patients with fracture neck of femur - a feasibility study
To describe the block dynamics and assess the analgesic efficacy of PENG block in patients with fracture neck of femur booked for operation, as an analgesic tool prior to positioning for spinal anesthesia or transfer to the operation table. minimizing pain is important because of humanity, also for hemodynamic control especially in patients in whom excessive sympathetic surge may result in an adverse cardiovascular condition. Patients could also cooperate better in positioning for spinal anesthesia if they are pain free. This allows easier and higher success rate of spinal anesthesia as this group of patients usually have degenerative spine with narrow intervertebral spaces.
单臂
Ⅳ期
N/A
N/A
威爾士親王醫院
/
15
/
2020-04-15
2021-06-15
/
Aged 18 or above, first episode of fracture of neck of femur scheduled for hip surgery, being mentally capable to understand the study protocol and to give consent.;
请登录查看contraindications to peripheral nerve block, for example coagulopathy or local infection, allergy to lignocaine;
请登录查看麻醉與重症監護部
/
求实药社2026-03-05
生物制品圈2026-03-05
医药观澜2026-03-05
医药观澜2026-03-05
河北省干细胞中心2026-03-05
生物制药小编2026-03-05
一度医药2026-03-05
炎明生物Pyrotech2026-03-05
药明康德2026-03-05
疑夕随笔2026-03-04