

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR1900026244
尚未开始
/
/
/
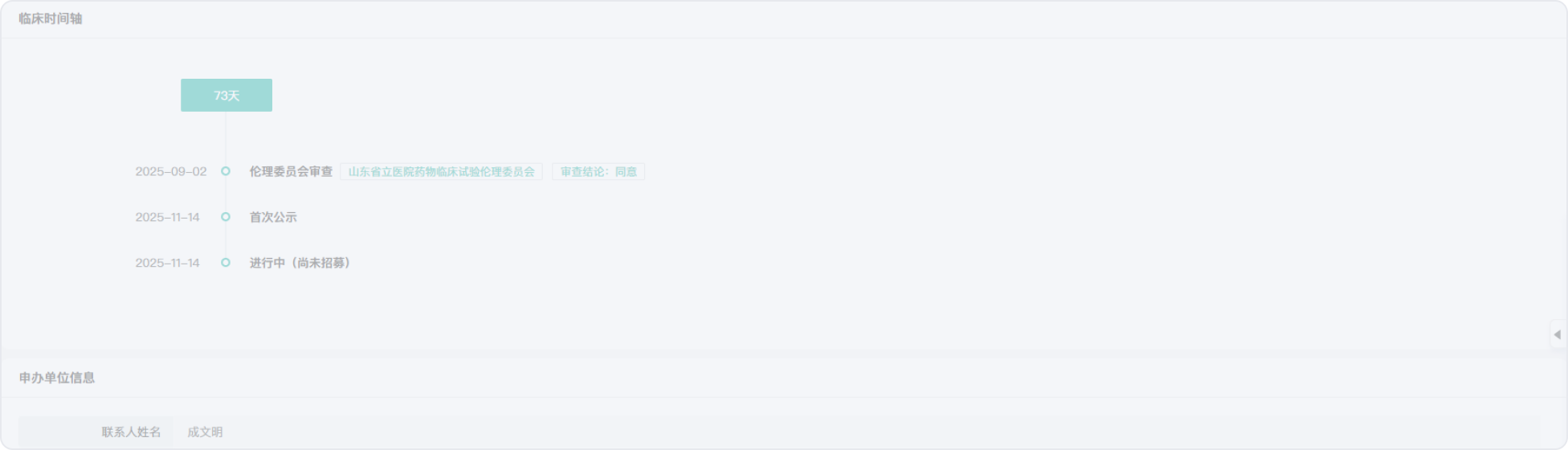
2019-09-28
/
/
乳腺癌
乳腺癌生存者自我管理應用程式的隨機對照試驗
Breast Cancer App protocol: a randomized controlled trial to evaluate a self-management app for breast cancer survivors
1) to examine whether there is the difference in the health literacy, problem solving skills, readiness of change, self-efficacy, self-management skill, and health-related quality of life between the intervention group and the control group; 2) to examine the usability of this self-management app versus a general health app.
随机平行对照
探索性研究/预试验
SPSS (IBM SPSS Statistics Version 25) will be used to generate the random sequence for group allocation.
Not addressed
Departmental research fund
/
244
/
2020-01-01
2021-06-30
/
1) female; 2) breast cancer survivors; 3) Cancer stages I to III; 4) completion of primary treatment within one year or longer; 5) aged 18 to 65 years; 6) acceptable literacy level (e.g. able to read, and write Chinese); 7) agree for checking of usage log of mobile app; 8) able to provide informed consent.;
请登录查看1) BCS diagnosed with severe mental illnesses; 2) Diagnosis of another cancer type rather than breast cancer; 3) has already participated in other clinical trial; 4) has been used other self-management app to manage the breast cancer.;
请登录查看香港理工大學康復科學系
/
求实药社2026-03-05
生物制品圈2026-03-05
医药观澜2026-03-05
医药观澜2026-03-05
河北省干细胞中心2026-03-05
生物制药小编2026-03-05
一度医药2026-03-05
炎明生物Pyrotech2026-03-05
药明康德2026-03-05
疑夕随笔2026-03-04