

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR-TRC-13003417
暂停或中断
/
/
/
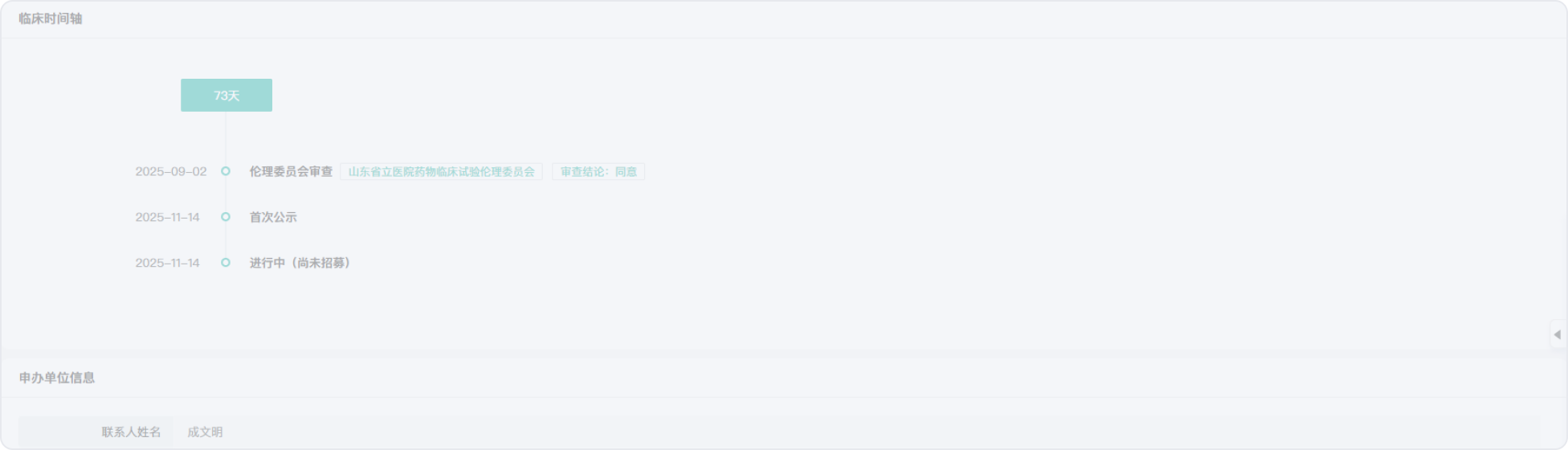
2013-08-06
/
/
卒中
機械手及經顱磁刺激聯合療法對中風病人手部運動功能康復的研究
機械手及經顱磁刺激聯合療法對中風病人手部運動功能康復的研究
機械手及經顱磁刺激聯合療法對中風病人手部運動功能康復的研究
随机平行对照
其它
randomly
/
NA
/
63
/
2013-07-01
1990-01-01
/
1) patients aged 18 or above; 2) first-ever ischemic subcortical stroke with onset time less than 21 days; 3) hand weakness as a result of stroke; 4) intracerebral hemorrhage excluded by brain computed tomography (CT) / magnetic resonance imaging (MRI); 5) the patient or his/her legally acceptable representative is willing to provide written informed consent.;
请登录查看1) personal history of epilepsy; 2) metallic implants in any part of body; 3) implanted cardiac pacemaker, defibrillator, medication pump or any other implanted electronics; 4) severe skull fracture, serious head injury, history of brain or spinal cord surgery; 5) brain tumour or other significant non-ischemic brain lesion on CT/MRI; 6) administration of drugs that potentially lower seizure threshold; 7) severe hand spasticity, open hand wound or hand deformity; 8) history of malignancy; 9) severe co-existing systemic diseases, severe dementia or psychosis; 10) inability to be followed up at regular interval; 11) pregnancy or breast-feeding.;
请登录查看Division of Neurology, Department of Medicine & Therapeutics, The Chinese University of Hong Kong
/
求实药社2026-03-05
生物制品圈2026-03-05
医药观澜2026-03-05
医药观澜2026-03-05
河北省干细胞中心2026-03-05
生物制药小编2026-03-05
一度医药2026-03-05
炎明生物Pyrotech2026-03-05
药明康德2026-03-05
疑夕随笔2026-03-04