

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR1900021998
正在进行
/
/
/
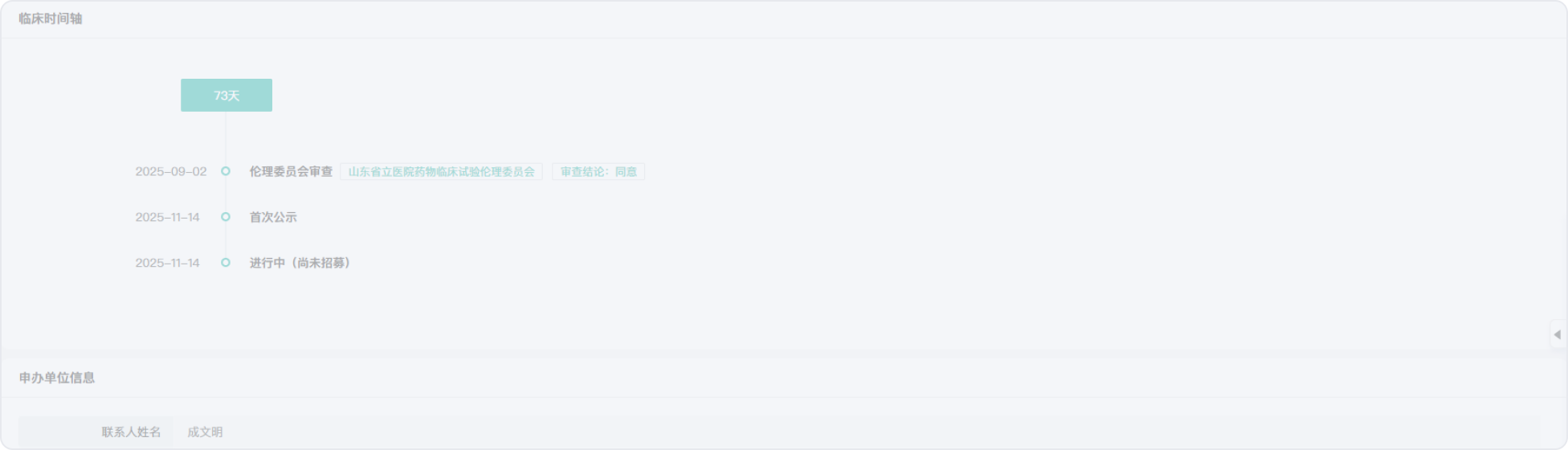
2019-03-19
/
/
青光眼
神經保護劑煙醯胺核苷治療青光眼的隨機對照試驗
神經保護劑煙醯胺核苷治療青光眼的隨機對照試驗
The study will randomize 125 patients with primary open-angle glaucoma (POAG) to receive an oral supplement of nicotinamide riboside 300 mg/day or a placebo (corn starch capsules) with an objective of comparing the rates of RNFL thinning (primary outcome measure) measured with a swept-source OCT over 24 months of follow-up between the treatment arms.
随机平行对照
其它
Patients will be randomized with minimization using a free open source computer program MinimPy to receive nicotinamide riboside 300 mg/day or a placebo (corn starch) at the baseline visit. Both the investigators and the patients will be blinded for the treatment assignment.
Both the investigators and the patients will be blinded for the treatment assignment.
Health and Medical Research Fund 2017
/
63;62
/
2019-04-01
2023-03-31
/
Age >=18 years; best corrected VA >=20/40; IOP <= 28mmHg; no history of laser procedure or intraocular surgery other than uncomplicated cataract extraction; patients with evidence of progressive RNFL/GCIPL thinning detected by Guided Progression Analysis(GPA) or Trend-based Progression Analysis (TPA);
请登录查看Pathological myopia; diseases that may cause visual field loss or optic disc abnormalities other than glaucoma; inability to perform reliable visual field; suboptimal quality of OCT images; and diabetic retinopathy/maculopathy; and a history of abnormal liver function.;
请登录查看Research Fund Secretariat, Food and Health Bureau, The Government of HKSAR
/
GMP办公室2026-03-04
抗体圈2026-03-04
上海和誉生物医药科技有限公司2026-03-04
BioArt2026-03-04
药明康德2026-03-04
药明康德2026-03-04
GLP1减重宝典2026-03-03
良医汇肿瘤资讯2026-03-03
良医汇肿瘤资讯2026-03-03
海创药业2026-03-03