

400-9696-311 转1

400-9696-311 转2

400-9696-311 转3

400-9696-311 转4





ChiCTR-IPR-15006587
结束
替比夫定
化药
替比夫定
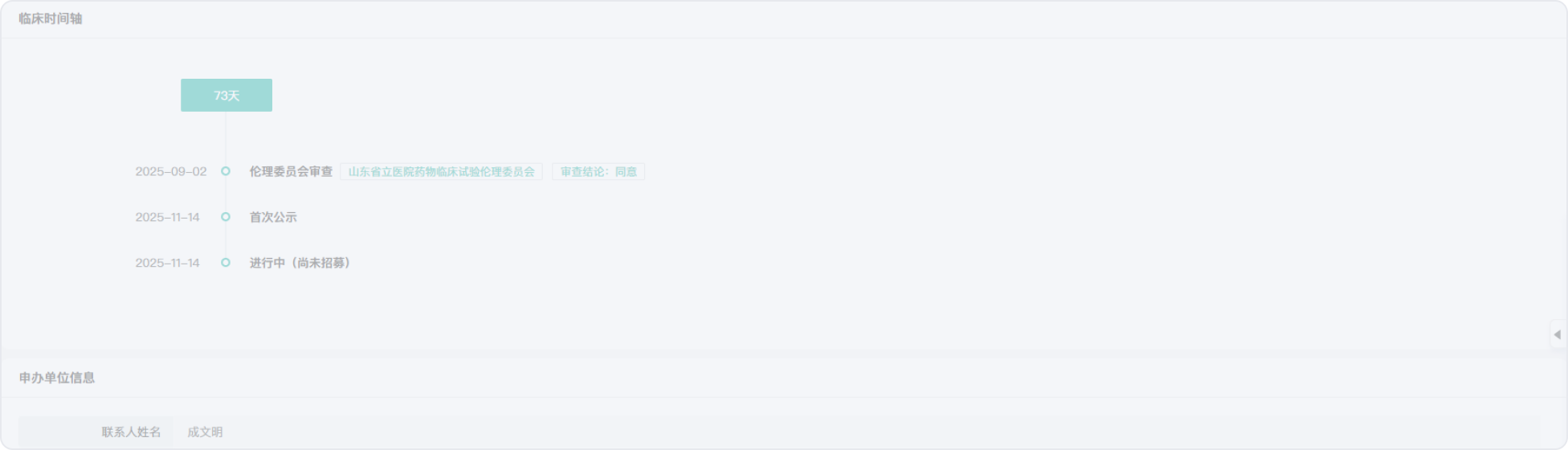
2015-05-26
/
乙肝相关肝癌
应用替比夫定抗病毒治疗对乙肝相关肝癌患者术后乙肝病毒再激活及预后影响的随机对照临床研究
应用替比夫定抗病毒治疗对乙肝相关肝癌患者术后乙肝病毒再激活及预后影响的随机对照临床研究
本研究的目的是对应用替比夫定抗病毒治疗预防肝癌患者术后乙肝病毒再激活的有效性进行评价。
随机平行对照
上市后药物
计算机生成随机号
/
东方肝胆外科医院和上海市科委支持
/
100
/
2008-11-01
2015-03-31
/
1. age 18 to 70 years; 2. positive test for Hepatitis B surface antigen (HBsAg) and negative test for antibody to hepatitis C virus (HCV-Ab) or human immunodeficiency virus; 3. serum hepatitis B virus-deoxyribonucleic acid (HBV-DNA) level lower than 2000 IU/ml; 4. Barcelona Clinic Liver Cancer (BCLA) stage 0, A or a solitary tumor with diameter> 5cm; 5. no extrahepatic metastasis; 6. no radiologic evidence of invasion into major portal/hepatic venous branches; 7. good liver function with Child - Pugh Class A and baseline serum alanine aminotransferase (ALT) level< 2 times the upper limit of normal (reference range<40 IU/L), with no history of encephalopathy, ascites refractory to diuretics, esophago-gastric variceal bleeding; 8. good renal function(a serum creatinine level < 133 umol/L); 9. no previous treatment for hepatitis B with nucleoside or nucleotide analogues or both; 10. no treatment with interferon or other immunomodulators within the previous 12 months; 11. a serum amylase or lipase level lower than 1.5 times the upper limit of normal; 12. no previous treatment of HCC; 13. negative resection margin (R0 resection); 14. histopathological result of the resected specimens being HCC. Eligible patients were excluded if they refused to participate.;
请登录查看拒绝参加者;
请登录查看第二军医大学东肝肝胆外科医院
/
锦波生物2026-03-03
品牌共创局2026-03-03
GMP办公室2026-03-03
医耀科技创新骑手2026-03-03
一度医药2026-03-02
药时空2026-03-02
贝达药业2026-03-02
摩熵医药2026-03-02
摩熵医药2026-03-02